What makes my baby blue?
That popular-song lament is also a question that's puzzled epidemiologists for the past half-century. Was it really rural well-water that made hundreds of infants literally turn blue? Why did the number of cases suddenly decline? As this saga by a member of the DMS faculty indicates, the annals of science will always, by their very nature, be incomplete.
By Roger P. Smith, Ph.D.

|
One can scarcely pick up a newspaper or magazine today without reading about another scientific triumph, from the cloning of a pig to a promising new drug for a terrible disease. There are, however, little backwaters of the human condition, where biological science has gone about as far as it can reasonably be expected to go but definitive answers are still lacking. This is the tale of one such hotly pursued but still unsuccessful effort to elucidate a medical mystery.
In the September 8, 1945, issue of the Journal of the American Medical Association, Dr. Hunter Comly, a pediatrician at the State University of Iowa, reported on two cases of a "previously unrecognized" condition that he feared might be confused with congenital heart disease. Dr. Comly wrote, "This condition may occur anywhere in rural areas where well-water is used in infant feeding." It came to be known variously as blue baby syndrome (the term for any condition that decreases the oxygenation in an infant's blood), well-water methemoglobinemia, or infantile methemoglobinemia.
|

|
|
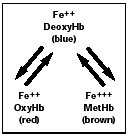
This diagram shows how changes in hemoglobin, and in the state of the iron in it, can change the color of the blood —from red to blue to brown. | |
How hemoglobin works
Following this story requires knowing a bit of chemistry. The hemoglobin in our red blood cells contains iron in a divalent (2+) state, which reversibly binds oxygen from the air we breathe. (See the diagram above.) Oxygen binding occurs in the capillaries of the lungs and forms oxyhemoglobin, which is bright red in color. The circulatory system carries oxyhemoglobin to all the body's tissues, where the oxygen is consumed in cellular respiration. The iron remains in a 2+ state, but as the oxygen is unloaded the oxyhemoglobin changes in color to the bluishpurple of deoxyhemoglobin. The difference in color between oxyhemoglobin and deoxyhemoglobin is readily apparent in humans as the difference in color between our arteries and our veins.
A number of chemicals, however, are capable of oxidizing the iron in hemoglobin to a trivalent state (3+). The iron basically rusts, which causes the pigment to change color—this time to brown or black, depending on how much of it is present in blood. This chemically altered pigment is called methemoglobin, and it has lost the ability to bind oxygen. If more than half of an individual's blood pigment is converted to methemoglobin, then oxygen transport to the tissues—especially the brain—becomes compromised, and signs of respiratory distress may be evident; the condition is potentially fatal.
|
Certain pathological conditions—such as asphyxia, drowning, or some lung or heart diseases— interfere in a different way with oxygen transport, causing abnormally high concentrations of deoxyhemoglobin to accumulate in the arterial blood. This condition results in a visible darkening of the skin and the mucous membranes, from pink to blue, a state that is called cyanosis (the word "cyanosis" comes from the Greek kyanos, meaning dark blue). The abnormal color is usually first noticed in the lips, and it spreads gradually to the fingers and toes, the face, and then the whole body.
Cyanosis is also evident when arterial and venous blood contains methemoglobin. When the concentration of methemoglobin in the blood of an adult exceeds 15% of the total hemoglobin, the individual becomes visibly cyanotic.
Normally, if humans are exposed to a single dose of a methemoglobin-generating chemical, such as sodium nitrite (also called just nitrite), the level of methemoglobin in their blood will rise sharply over an hour or two—the rate of rise depending on the dose. It then reaches a plateau and declines over several hours back to normal. This reversal of methemoglobinemia is due to the spontaneous activity of a reducing enzyme found in red blood cells. Thus a built-in system exists for correcting acquired methemoglobinemia, although the system has its limits. It can be overwhelmed by very large doses of oxidizing chemicals, which can generate lifethreatening levels of methemoglobin more rapidly than the enzyme is able to reduce them. The end result may be death. (See the adjacent diagram.)
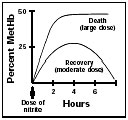 The body can recover from a moderate dose of nitrite, clearing the methemoglobin it generates from the blood. But a large dose can overwhelm the system and lead to death. |

|
|
The family lived on a farm and got their water from a well. Samples of the well-water were found to contain high concentrations of nitrate and smaller amounts of nitrite. | |
Fortunately, simple laboratory tests can quickly reveal whether cyanosis is due to the presence of deoxyhemoglobin or of methemoglobin. This distinction is important, because the corrective action differs in the two conditions. The administration of oxygen and other measures to correct the specific problem are indicated in a case of excessive deoxyhemoglobin. Oxygen, however, does nothing to address the underlying cause of methemoglobinemia —namely, the inability of the hemoglobin to bind oxygen.
It was this confusion that Dr. Comly was concerned about. He thought the cyanosis of infantile methemoglobinemia might be confused with cyanosis due to excessive deoxyhemoglobin, caused by congenital heart disease. The most common reason for infantile cyanosis is a structural defect in the heart that allows some deoxygenated blood returning to the heart to be pumped right back out before going to the lungs to be oxygenated—a condition that can be cured by surgery. Dr. Comly wanted to ensure that no infants suffering from the rarer chemically induced version of blue baby syndrome were misdiagnosed.
The first cases
Dr. Comly's original report is worth careful study; it was such a masterpiece that the entire paper was reproduced in the same journal 42 years later. The circumstances surrounding these two initial cases were typical of many other reports to come. In his first case, a white baby girl was delivered by cesarean section. She left the hospital on the 12th day after her birth in good health. The family lived on a farm and got their water from a well.
At age 33 days, after a long period of irritability, she was noted to be drowsy, diarrheic, and alarmingly cyanotic. She was rushed to the local hospital. Laboratory tests showed that she had methemoglobinemia. She was injected with methylene blue, a drug used to treat methemoglobinemia, and within 30 minutes her color, respiration, and heart rate were normal. She was discharged after a week.
In two days, however, she was readmitted with similar symptoms. The same treatment resulted in a good response, and this time she was discharged after 16 days.
Just three days later, she was again readmitted in worse condition than ever. This time it took two hours for the methylene blue to restore her color. By now her doctors realized that a significant change in the baby's environment from hospital to farm was the water used to make her formula. The parents were warned not to use any more of their wellwater, and the baby was put on acidified whole milk.
The father, however, was not satisfied with this incomplete explanation, and he made arrangements to have his daughter referred to Dr. Comly. His physical examination of the baby and routine laboratory tests showed no abnormalities. An analysis for methemoglobin in her blood yielded only a "high normal" value. Samples of the wellwater brought along by the father were found to contain high concentrations of nitrate (NaNO3) and very much smaller amounts of nitrite (NaNO2). The water was also highly polluted with coliform bacteria. Dr. Comly saw the baby again for follow-up when she was four months old and found her to be completely healthy.
The second case on which Dr. Comly reported in that 1945 article was similar in all key respects.
Nitrates and nitrites
Dr. Comly suspected that the nitrates in the family's well-water were at fault. There was a problem with blaming blue baby syndrome on nitrates, however, as nitrates never cause methemoglobinemia. But nitrite, as opposed to nitrate, is a potent methemoglobin- generating agent—a fact that was known then. These two chemicals are very easily confused because there is only a one-letter difference in their names and only a one-oxygen difference in their formulas. But there is a world of difference in their toxicity. Nitrate is benign and nitrite is potentially deadly. It was also known at the time that nitrates could be converted to nitrites by bacteria, including some that are found normally in the human bowel. (See the diagram at right.)
Therefore, Dr. Comly reasoned that in these two little patients, nitrate had been converted to nitrite by bacteria in the intestine. The nitrite, he presumed, was then absorbed into the blood, where it reacted with hemoglobin to generate methemoglobin. He felt that the traces of nitrite already present in the well-water were insignificant.
This hypothesis, however, did not explain why the rest of the family was not affected. The role of the baby's diarrhea also remained something of a mystery. Whether the nitrate salts or something else in the polluted water was responsible, or whether this problem had an entirely different cause, remained unknown. Only one blue baby who came to Dr. Comly's attention did not have diarrhea.
Before publishing his report, Dr. Comly collected from colleagues anecdotal accounts of 17 more cases, including one that had resulted in death. It appeared to him that "the condition was not rare." Indeed, a mini-epidemic was soon underway.
Exploring the epidemiology
In the August 1951 issue of the American Journal of Public Health, Dr. Graham Walton, a sanitary engineer, published a benchmark examination of blue baby syndrome. He reviewed the worldwide medical literature on the subject up to that point and summarized the results of a survey that had been conducted in 1949-50 by the American Public Health Association.
The Public Health Service questionnaire from which Dr. Walton compiled his results had been sent to 48 states, Alaska, and Hawaii, and all but one state responded. The survey turned up 278 cases and 39 deaths. However, physician-reporting for this disease was not mandatory, so the true number of cases may have been substantially larger. Most of the U.S. cases occurred in the north central states, and similar cases were reported in the literature from Canada and Europe.
Field investigations revealed that the waters with high nitrate content came from wells that generally were old; were dug rather than drilled; had permeable casings; and were poorly covered, so that surface water could enter freely. In all the reported blue baby cases, the wells were located near barnyards and pit privies. Most were highly contaminated with coliform bacteria. The nitrate content varied from 64 to 140 parts per million (ppm), and the severity of the infants' symptoms seemed to parallel the amount of nitrate present.
No cases turned up by the survey were associated with the consumption of water with a nitrate content of 10 ppm or less, and only 2.3% of the cases were associated with 20 ppm or less. The principal sources of the nitrate in well-water were believed to be decomposition products of plants, animals, and microorganisms; liquid and solid animal wastes; and nitrate fertilizers. Although municipal water works in five states reported a nitrate content in excess of 10 ppm, no cases were ever ascribed to municipal water supplies.
In addition to methemoglobinemia and diarrhea, reports in the interim suggested that some affected infants had an elevated gastric pH. The investigators who found a more alkaline environment (alkaline meaning a high pH) in the stomachs of some infants postulated that this condition might be more hospitable to coliform organisms than the stomach's normal acidic environment, permitting them to survive higher up in the gastrointestinal tract than usual. There would thus be more opportunity for them to reduce nitrate to nitrite before the nitrate was absorbed into the blood. Under ordinary circumstances, nitrate would be absorbed before coming in contact with bacteria growing further down in the bowel. The investigators further postulated that by about one year of age, the pH level of an infant's stomach drops to nearly that of adults and is then a significant deterrent to bacterial growth in the intestine. This would at last explain why most cases occurred in infants under six months of age.
In the U.S., reports of new cases fell off steadily through the early 1950s, without any corrective action having knowingly been taken. Today, the disease has all but disappeared. Only two cases have been reported since the mid-1960s. The epidemic waned as suddenly as it had appeared.
However, the concentration of nitrates in some Midwestern water supplies continues to increase, probably reflecting increasingly heavy use of nitrate fertilizers. Some communities in the Midwest now have difficulty meeting the 10 ppm standard during the growing season. Residents have sued authorities, not on the basis of alleged adverse health effects, but on the grounds that the "nuisance" of a water supply not in compliance with standards lowers their property values.
The hypothesis of gastrointestinal reduction of well-water nitrate to nitrite has held the consensus position among scientists for nearly 50 years, but there are aspects of the process that are incompletely understood and, therefore, troubling.
This hypothesis explains why the disease occurred only in newborns, why it was associated with the consumption of contaminated well-water, why a change in water supply alone was sufficient to alleviate the condition, and why there was a relationship between the severity of the illness and the nitrate concentrations in the water. But it does not explain why the cases stopped appearing in the late 1950s or why no cases were associated with municipal water supplies.
While it is difficult to study a disease process during its course, it is impossible to study an epidemic that no longer exists. It is likely that what is known today about well-water methemoglobinemia will remain the extent of our knowledge.
Drinking water standards
In 1962, the U.S. Public Health Service recommended a national nitrate standard of 10 ppm. The recommendation became a requirement upon the passage of the Safe Drinking Water Act of 1974, which mandated that the Environmental Protection Agency (EPA) establish federal standards to protect humans from harmful contaminants in drinking water; the EPA endorsed the 10 ppm limit. The law also authorized the EPA to seek the expertise of the National Research Council (NRC) to identify the health effects of specific contaminants. A 1995 NRC document, "Nitrate and Nitrite in Drinking Water," was the most recent of the required periodic reviews and again upheld the 10 ppm nitrate standard.
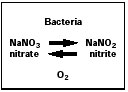
This diagram represents the fact that some bacteria can convert nitrate, a benign substance, into deadly nitrite. But whether this action was responsible for the 1950s epidemic of infantile methemoglobinemia is less certain. |

|
|
The major source of nitrate exposure in the diet of U.S. adults is not water but vegetables. However, adult vegetarians are not recognized as being prone to methemoglobinemia. | |
The thinking behind this standard is that it protects a very specific subgroup of the U.S. population—namely, infants under six months of age who are fed formula prepared with well-water. No other subgroup has been identified as being at particular risk. The fact that the condition has all but disappeared strongly suggests that the standard is adequately protecting the at-risk group. So were it not for this emotionally compelling subgroup, the standard could probably be set higher —although how much higher would be difficult to ascertain.
A single startling fact supports this assertion. It is well known that the major source of nitrate exposure in the diet of U.S. adults is not water but vegetables. Drinking water makes up only 3% of the total dietary intake of nitrate, whereas vegetables account for 86%, with fruits, juices, cured meats, and other foods making up the balance. Vegetarians consume about four times as much nitrate as do omnivores, but adult vegetarians are not recognized as being prone to methemoglobinemia. Thus the contribution of water-borne nitrate to the total adult intake is inconsequential.
If there were a widespread effort to educate parents and physicians to avoid nitrate-rich water in infant formula, or if the families of at-risk babies were provided with bottled water, it is possible the drinking-water standard could be doubled without seriously compromising human health. This could save millions of dollars.
In fact, in the July 1999 issue of Environmental Health Sciences, Dr. Alex Avery of the Hudson Institute's Center for Global Food Issues called for a relaxation of the EPA drinking-water standard for nitrates (though not for nitrites). Dr. Avery's action was prompted in part by a new series of cases beginning in the early 1980s. At that time, scattered reports began to appear of infantile methemoglobinemia that were clearly not due to nitrates in either food or water. Fairly compelling evidence accumulated suggesting that these cases were due to the generation of nitrite endogenously, or within the affected individual's body; there are pathways by which this could theoretically occur. To date, several hundred cases have been identified.
Dr. Avery posits that the scientists of 50 years ago were misled by the presence of nitrates in wellwater. He believes the nitrates were a red herring and that all of the older cases were due to this more recently discovered phenomenon. If he is correct, there would be no reason at all to adhere rigidly to the 10 ppm nitrate standard.
The dilemma
So the toxicologists of today have to decide whether to believe the admittedly incomplete science of 50 years ago or the evidence about this newer phenomenon—which is, so far, even more incomplete.
Even though science was much more primitive 50 years ago, I have more faith in the observations and skills of that day than Dr. Avery does. And there are other reasons for keeping the 10 ppm standard. A doubling of the drinking water standard for nitrate would inevitably lead to a rise in the nitrate concentration in surface waters. Contamination of surface waters, however, would be a minor problem compared to the likely eventual contamination of groundwater. Groundwater exists as aquifers, highly permeable underground strata, analogous to underground rivers. Most municipal water supplies are drawn from artesian aquifers; indeed, 97% of all fresh water on earth is in aquifers. The cost of decontaminating groundwater, because of its sheer volume, is likely to be many orders of magnitude above the cost of decontaminating surface waters. There are far worse chemicals that could be contaminating aquifers, but nitrate shares with the most undesirable of them the property that it is stable. Once there, it would stay for a long time.
An even more recent chapter in this story involves frogs. There are two parts to this chapter. The first is a widely reported increase in the incidence across the United States of frog deformities, such as twisted, missing, or extra legs. This has been blamed variously on ultraviolet light penetrating a thinning ozone layer, pesticides, and, most recently and strongly, parasitic flatworms. The presence of nitrates in ponds and surface waters could indirectly foster the multiplication of these parasites. The second part of the frog chapter has to do with a worldwide decline in the number of amphibian species. Collaborating groups in Spain and Oregon have again pointed the finger at nitrites and nitrates. Although there is a significant difference in sensitivity among various species, some frog larvae are sensitive to 1 ppm of nitrite, which is the EPA standard for drinking water, and some exhibit high rates of mortality at 5 ppm, which is the standard for protection of warm-water fishes. And the recommended nitrate level for warm-water fishes of 90 ppm is highly toxic to some species of frog larvae. Again the science is incomplete, but this evidence supports upholding the 10 ppm nitrate standard.
We are thus caught on the horns of multiple dilemmas. Any restriction on the use of nitrate fertilizers in America's breadbasket would translate directly into decreased food supplies and increased costs. Removing nitrate from drinking water is expensive, but not nearly as expensive as it would be to remove it from surface water sources and, eventually, from aquifiers.
The relationship, if any, between well-water methemoglobinemia and the new non-nitrate cases of infantile methemoglobinemia is poorly understood. If the nitrate standard were raised, would we just assume that Dr. Avery's theory was correct and Dr. Comly's wrong? Or would we hedge our bets by providing bottled water to families with at-risk newborns (which would be costly and cumbersome) or undertake an educational effort about the dangers of nitrate-rich well-water for infants (which would be difficult to implement with sufficient thoroughness). And the argument that methemoglobinemia is easily recognized and treated is problematic, since it depends entirely on the ability of individual physicians to recognize a rare condition and act accordingly.
So hard choices have to be made on the bais of incomplete information. It behooves society to proceed with caution.
Roger Smith is the Irene Heinz Given Professor of Pharmacology and Toxicology at DMS and an adjunct professor of environmental studies at Dartmouth College. A member of the DMS faculty since 1960, he writes regularly for Dartmouth Medicine.
Roger Smith, Medical Detective
Dartmouth toxicologist Roger Smith, the author of the adjacent article, has had a career-long interest in nitrates, nitrites, and methemoglobinemia. His own role in those elements of the "blue baby" saga was not a central one, however.
Nevertheless, he was impelled to relate the story both because of his tangential connections to it and because of two other factors. First, he felt that the tale illustrated some important points about the course of science. And second, he has long had a fascination with "medical detective" stories.

|
|
This portrait of "medical detective" Roger Smith is the work of one of his former toxicology department colleagues (and his predecessor as the Irene Heinz Given Professor)—Robert Gosselin, M.D., Ph.D. (bio) |
Smith's connection with methemoglobinemia goes way back to the very beginning of his career. The condition—which results in a blue discoloration of the skin—isn't always life-threatening. Some Alaskan Eskimos and certain people from Appalachia are "naturally" blue because they were born with a deficiency of the enzyme methemoglobin reductase. About the time Smith arrived at DMS, in 1960, he became intrigued with methemoglobinemia's potential as an antidote for hydrogen sulfide poisoning. Physicians had known since the 1930s that sodium nitrite, which induces methemoglobinemia, was an effective antidote for cyanide poisoning. "Cyanide and sulfide have chemical and biological similarities, so it seemed possible that methemoglobinemia might be antidotal in hydrogen sulfide poisoning [too]," Smith says.
Hydrogen sulfide, a rapidly acting and violently poisonous gas that smells like rotten eggs, can be found in petroleum drilling sites, sewers, tanneries, kraft paper mills, liquid manure facilities on farms, heavy water plants, and privies. "OSHA [the Occupational Safety and Health Administration] says that hydrogen sulfide is the leading cause of sudden death in the workplace," Smith explains.
The antidote for cyanide poisoning did indeed work for hydrogen sulfide. An injection of sodium nitrite temporarily converted some hemoglobin to methemoglobin, which in turn trapped some of the free sulfide in the blood as sulfmethemoglobin, pulling sul- fide out of the tissues.
Smith has been called in as a consultant to communities where fatal hydrogen sulfide poisonings have occurred and often had to reassure panicky citizens that they were not in danger. Hydrogen sulfide gas is "a poison that is violently toxic in a single large dose, but [it is] not an environmental problem at lower doses," he says. "The Public Health Service says you can be exposed to 10 parts per million hydrogen sulfide, eight hours a day, five days a week, over your entire lifetime, without adverse effect."
Before joining the DMS Department of Pharmacology and Toxicology 40 years ago, Smith received his B.S., M.S., and Ph.D. degrees from Purdue University and worked for three years in the Forensic Toxicology Section of the U.S. Army's European Medical Laboratory in Landstuhl, Germany. He was chair of pharmacology and toxicology at DMS from 1975 to 1987 and an adjunct professor at Vermont Law School from 1981 to 1987, and he has been an adjunct professor of environmental studies at Dartmouth College since 1984 and the Irene Heinz Given Professor of Pharmacology and Toxicology since 1993. He has also served on a number of national committees including, in 1969, an ad hoc advisory group to President Nixon on nitrates, and, in the mid- 1990s, the National Research Council subcommittee that conducted the most recent review of acceptable levels of nitrates and nitrites in drinking water.
Smith and fellow researchers have also investigated how nitric oxide vasodilators —nitrite, nitroprusside, azide, hydroxylamine, and nitroglycerin—are converted to nitric oxide, and they have studied the toxic effects of sodium azide, the active ingredient in automobile airbags.
Although Smith will formally retire this year, he plans to continue teaching at DMS and at the Institute for Lifelong Education at Dartmouth (ILEAD)— as well as writing articles for Dartmouth Medicine. He teaches two versions of a course titled "Medical Detectives"—one for medical students and one for ILEAD participants. The course is based on the work of one of his favorite writers, Berton Roueche, whose "Annals of Medicine" essays appeared in The New Yorker magazine over a 40-year period and were later published in book form. With the help of Dartmouth medical students, Smith has brought many of Roueche's stories up-to-date in terms of current science and medical practice; his addendums to these stories can be found at http://www.dartmouth.edu/~rpsmith/index.html.
"I am fond of telling people that they [Roueche's stories] played an important role in my choosing a career as a biomedical scientist, but I can't really be certain of that now," Smith writes on his Web site. "One of my favorites was 'Eleven Blue Men.' Perhaps it was only a coincidence that I was to spend 40 years at Dartmouth Medical School exploring related areas." And is it only coincidence as well that he is now writing about blue babies?
Laura Stephenson Carter Laura Carter is the associate editor of Dartmouth Medicine magazine.
Back to Dartmouth Medicine Summer 2000
