Intimate Strangers
The wonders and mysteries of the world of microbes are explored in a new book coauthored by the former chair of biochemistry at DMS. Here are some excerpts from the just-published work.
By Cynthia Needham, Mahlon Hoagland, Kenneth McPherson, and Bert Dodson
Imagine that, like Alice, you stepped through the Looking Glass and became small—VERY small, in fact—1/200,000th of your normal size. The world around you would look very strange indeed, like some planet elsewhere in the universe, a land filled with odd inhabitants and mysterious forms and shapes.
There are lavender feather boas, a foot or more long, that twist and undulate past you, clearly intent on reaching some unknown destination. Swirls of green and purple pillow-sized blimps propel about, slowing to nudge you, then engaging their long, propeller-like appendages to dart out of view. A glistening creature slides up, its changing shape seeming to envelop its surroundings in transparent ooze. It stops as though to look you in the eye and say good day, then moves on, leaving no trace behind.
The inhabitants of this strange and tiny world are microbes—bacteria, fungi, viruses, protozoa, and algae. Most people think of them as germs, but these intimate strangers are much more than that. They are the force that keeps our planet working.
Microbes were here long before we were. We have traced the whispers of their evolutionary history back more than 3.5 billion years. All of life's diversity evolved from their simple, single cells. But they are not entirely benign. Although most are harmless, a few can turn against us, causing disease in us and in our plants and animals. Others are familiar enemies with which we have learned to live. And a very few are lethal strangers that can overwhelm us unless we can mount a swift and powerful defense.
In spite of their almost four-billion-year history on earth, our discovery of microbes is very recent. Knowledge of their existence came only after we had magnifying devices that allowed us to look into their unseen world—to step through the Looking Glass. In the mid-17th century, a Dutch amateur microscope builder, Antony van Leeuwenhoek, was amazed and delighted when he first saw these "wee animalcules."
Over the last half of the 20th century, our knowledge of this unseen world has expanded exponentially. Now scientists are racing to learn the secrets of microbes' vast and interconnected communities—communities that make our life on earth possible—before we so disrupt their environment that they change the face of our planet again.
Denizens of the Microbial World
The microbial world includes all living organisms that can perform the basic functions of life—metabolism, reproduction, and adaptation—as single-celled creatures. We know these creatures as bacteria, archaea, algae, fungi, and protozoans. This world also includes a striking anomaly—a string of nucleic acids that relies on other cells to handle the chores of everyday life. We know this ne'er-do-well as a virus.
Millions of microorganisms can fit into a teaspoon of seawater, but the microbial community makes up more than half of life's total mass. An individual microbe has virtually no impact on its community, but collectively, microbes have shaped the face of our planet. At this point in time—unless somebody has again redrawn the tree—scientists use common biological traits to assign these players to one of three main groups.
Bacteria and Archaea (a.k.a. the Prokarya)
The Bacteria and Archaea are the smallest independent living cells. They are on average 50 times smaller than our cells and 70 times larger than viruses. These organisms share a common structural feature—they lack a true nucleus. Their genome consists of a single molecule of double-stranded DNA. The total number of genes in an organism is a measure of its complexity, i.e., the number of proteins it needs to do its thing. The prokaryotes' five million nucleotides equal about 5,000 genes. Humans, by comparison, have some 80,000 genes.
A membrane similar to that in our cells surrounds the bacterial cell. Outside this membrane is a tough cell wall made of protein and carbohydrate. Many members of the Bacteria and Archaea have whiplike extensions (called flagella) that rotate, propelling them through their watery environment like an outboard motor. Bacteria and Archaea reproduce without sex, by dividing in half.
Bacteria generally reside in places familiar to us: for example, soil, water, food, and animal skin and digestive tracts. The Archaea, in contrast, are often found in what we would consider the harshest of conditions—extremes of heat and salt, highly acid or highly alkaline environments—places where most other life forms would fear to tread.
Algae, Fungi, and Protozoa (a.k.a. the Microbial Eukarya)
The microbial Eukarya are organisms that all contain a true nucleus. They are considerably larger, more complex, and more varied structurally and functionally than the Prokarya. Their DNA is spooled on proteins in structures called chromosomes and is harbored in their membrane-encased nucleus. Eukaryotic DNA contains some 10 times the number of genes found in the Prokarya.
Eukarya mostly live as single cells, but some form colonies and some are truly multicellular. Kelp, for instance, is made up of permanent colonies of algae, and mushrooms are differentiated aggregates of fungus cells. Algae and fungi are surrounded by tough cell walls similar to the Bacteria and Archaea. Protozoa such as amoebae and paramecia are more animal- like than plant-like. Unlike algae and fungi, they lack rigid cell walls and are able to move about by various means and capture their food. Certain microbial Eukarya form the masses of microorganisms in the oceans, called plankton, that are a critical link in the food chain.
Viruses
Viruses are not cells. They are packages of DNA (or sometimes RNA) containing relatively few genes in a protein shell. They are roughly 2,000 times smaller than an animal cell. They can infect virtually all types of cells—bacterial, fungal, protozoan, plant, and animal.
Viruses can exist outside cells for long periods in a dormant state, but can only reproduce inside living cells, subverting the cell's machinery for their own use. They may multiply to large numbers, destroying the host cell and causing disease. Others may enter and leave cells without causing trouble. And sometimes they leave a few of their genes behind. They may also incorporate a gene from their host into their own genomes and then deposit the gene in other cells they infect.
Living in a Microbial World
We are never alone. We have literally billions of microbes living in us and on us. But they aren't really in us. They inhabit our skin, our mouths, our digestive tracts—colonizing every surface that has access to the outside. In fact, from the microbe's perspective, we are a huge hollow tube, carrying them from place to place and keeping them nourished. These microbes are key players in our amazing, intricate, and dynamic personal ecosystems. We rely on these ecosystems—our "normal flora"—to keep us healthy.

|
|
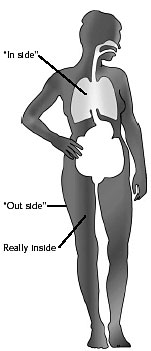
A tube has an "in side" and an "out side." |
|
|
Microbes residing on our skin, mouth, and intestinal tract are on both the "out side" and the "in side" of our tube. Microbes invading muscle, bone, and internal organs are REALLY inside. |
Although our partnership with this normal flora is usually mutually beneficial, the relationship can turn adversarial. The natural drive to get more and do better is as common in the microbial world as it is in our own. From our viewpoint, we prefer that microbes stay in our hollow tube, outside the sterile tissues that make up our body. From their viewpoint, the grass may appear greener on the inside of the tube.
This difference of outlook can sometimes breed contention. Even the friendliest microbe turns dangerous if it breaches our skin barrier and relocates in our muscle, blood, or bone.
We have evolved a set of defenses to keep microbes on their side of the line. Our skin and mucous membranes form a barrier between what is really inside us and what is really outside us. Fluids bathing our body surfaces—like tears, saliva, and stomach acid—contain enzymes and other compounds harmful to many microbes. Cells, functioning like a well-trained border patrol, recognize foreigners when they step across the line from outside to inside. The border patrol cells link to a set of internal defenses that build better and better protection with each new encounter.
We each differ in our defensive repertoires and our ability to effectively employ them. Some people get influenza while their office mates don't. Our genetic makeup and many additional factors—nutrition, stress, possibly even our psychological well-being—can build or weaken our defenses.
Microbes have evolved their own strategies for living with us. The microbes in our normal flora have muted their invasive qualities and are generally held in check at our boundaries. Only rarely do they cross the line and turn into dangerous friends, and our defenses are exceedingly good at evicting them from forbidden spaces when they do.
When Friends Turn Against Us
Our microbial partners join us from the moment we are born. They come from the first people we encounter—our mother, father, nurses and doctors, sisters and brothers. Over a matter of hours, we become home to huge numbers of microbial companions. They and we have begun an intricate partnership, an association that will continue for the rest of our lives.
Some of these microbial inhabitants remain steady partners. They stick to the various surfaces of our body, using specialized attachment sites that seem to have evolved just for the purpose. Others are passing through on their way to someone or somewhere else. Our microbial partners come and go as the years pass and as we change, but we will never be without a huge family of microbes.
Most of the time we live with these microbial inhabitants in a mutually beneficial relationship, exchanging valuable resources. Some of our steady partners make nutrients that we rely on for our health and well-being. Some produce substances that are noxious to other, less familiar microbes, protecting us from invasion. In exchange for their services, we supply them with a steady diet and a nice warm place to live.
Among the microbial members of our normal flora are some skilled opportunists, microbes just waiting for a chance to get an advantage. We can provide that chance through our own actions—enhancing a microbe's food supply, disrupting the normal microbial competition, or making a hole through skin or mucous membrane barriers—all enticing opportunities for members of our normal flora. Other microbes are less aggressive, but even a normally benign microbe can get out of hand, teasing the immune defenses of our border patrol into damaging our own tissue in the ensuing exchange.
|
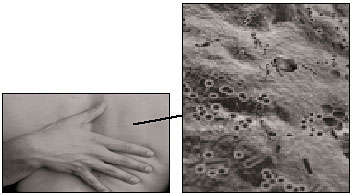
Propionibacterium |

|
|
Identity: Bacterium |
|
|
Our skin harbors at least 30 different kinds of microbes. On our backs there may be several hundreds to thousands per square centimeter; on damper surfaces, thousands of times that many. |
Familiar Enemies
Species that are closely intertwined tend to evolve toward cooperation. Over time, even putative enemies tend to settle into some form of accommodation. They become so familiar that enemies can almost appear as friends.
An Enemy That Is Usually Just Passing Through
Consider Salmonella typhi. This bacterium causes typhoid fever, a disease that is very different from the intestinal upset we get from other Salmonella species. After thousands of years of contact with us, S. typhi has come to depend on us for survival. It doesn't infect other animals. Across the millennia, the bacterium has learned enough about us to be a skilled invader, sending misleading chemical messages to our cells and gaining easy entrance. We, in turn, have learned something about mitigating the bacterium's lethal properties.
We become infected with S. typhi by eating contaminated food or drinking contaminated water. Once S. typhi has survived its journey to the small intestine, the bacterium uses its small, whiplike flagella to swim close to the intestinal wall, all the while monitoring its environment. When conditions of pH and oxygen concentration are just right, it pushes a protein through its outer surface. The protein becomes its docking device. Along the inside surface of the small intestine are specialized cells called M cells. They have sites on their surface that the bacterium's docking protein just fits. As the bacterium touches down and locks on, it signals to the cell, perhaps saying, "Hi. I'm food. It's dinner time!" The M cell, misled by the greeting, responds by ruffling its outer membrane, reaching out, and drawing the bacterium inside. Once inside the cell, S. typhi begins to reproduce rapidly, free from the pressures and competition in the intestine.
From here, the plot thickens. The newly minted bacteria break free from the M cell and invade deeper into the tissues surrounding our small intestine. They send out a new message, saying, "I've slipped past your defenses, so come and get me if you can."
S. typhi's taunt reaches the cells that patrol our tissue for invaders. We call these cells—part of our immune defenses— macrophages. Macrophage means "big eater," and the name is apt. Macrophages scout constantly, picking up foreign material and microbes that have wandered into the wrong spots in the body. Normally, they ingest intruders, subjecting them to a barrage of toxic chemicals and bringing them to a horrible death.
Not so for S. typhi. Having "heard" the chemical taunt, the macrophage reaches out and gobbles up the bacteria. S. typhi now changes its tactics, convincing the macrophage to forgo its normal malevolent behavior. The bacterium not only survives but also thrives, safely reproducing yet again inside the macrophage.
The infected macrophages return to the bloodstream, carrying S. typhi along with them. From there the bacteria can invade new macrophages in the spleen and liver, getting food and reproducing all the while.
S. typhi causes us considerable discomfort, but our immune system—or the antibiotic we take—usually kills the bacteria. By then, however, the bacteria have successfully reproduced, some going on to infect other susceptible people. This is typical of an encounter with the most familiar of enemies—the result of messages sent between two old adversaries who have coevolved and learned to coexist.
The Salmonella Journey
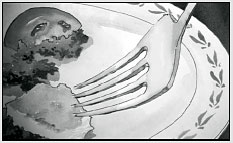
1. S. typhi usually begins its trip through us when we eat contaminated food or water.
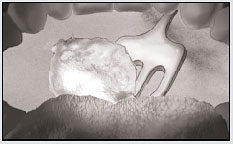
2. The bacteria survive our first line of defense— digestive enzymes in the saliva.

3. S. typhi must still struggle through the extreme acid conditions in our stomach.
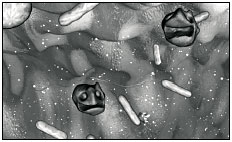
4. After that acid test, it faces an onslaught of bile and other chemicals in the upper small intestine.

5. S. typhi now moves quickly from "in side" to inside, entering specialized cells, called M cells, that are part of the wall of the intestine.
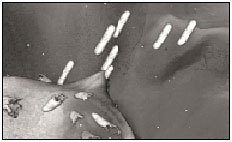
6. The invading bacteria multiply inside the M cells, then penetrate the tissue adjacent to those cells. Now they're REALLY inside.
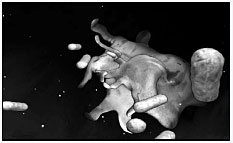
7. Here the bacteria encounter macrophages roving among the cells surrounding our intestine and are gobbled up.

8. The macrophages would normally kill bacteria, but S. typhi chemically stuns the macrophage's killing device and continues to multiply.
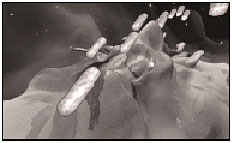
9. The macrophages return to the bloodstream, where the bacteria break out to infect other cells, and we get typhoid fever!
Typhoid Mary
Salmonella typhi may only be a familiar microbe to anyone who has recently traveled into a developing country. But back at the beginning of the 20th century, you didn't need to travel to find it. You could get it with your meal at the local restaurant.
 The now immortalized "Typhoid Mary"
Mallon was a professional cook in New
York City at the turn of the century.
After several outbreaks of typhoid
fever in the City, the
local health officials, operating
in the fine tradition of
John Snow, the first infectious
disease detective,
traced the outbreaks to
Mary, who was ultimately
identified as a chronic carrier
of S. typhi. She had been serving
her customers the microbe along
with her cuisine.
The now immortalized "Typhoid Mary"
Mallon was a professional cook in New
York City at the turn of the century.
After several outbreaks of typhoid
fever in the City, the
local health officials, operating
in the fine tradition of
John Snow, the first infectious
disease detective,
traced the outbreaks to
Mary, who was ultimately
identified as a chronic carrier
of S. typhi. She had been serving
her customers the microbe along
with her cuisine.
She was offered a solution. The microbe lives in the gall bladder in chronic carriers, and removal of the infected gall bladder was the only remedy then known. However, Mary both refused to have the operation and refused to cease cooking, at which point the constabulary promptly arrested and incarcerated her. After three long years, Mary agreed never to cook professionally again, and she was released.
But Mary apparently loved cooking too much to be able give it up. She immediately changed her name and resumed her profession. Typhoid Mary was employed by a variety of hotels, restaurants, and hospitals, from which she managed to spread S. typhi to many more people before she was finally tracked down once again. She spent her remaining years quarantined in a New York City hospital.
John Snow: The First Disease Detective
John Snow is viewed by many as the father of epidemiology, the approach used by modern public health services to track disease. Snow, the son of a farmer, was apprenticed to a Newcastle surgeon when he was 14. In 1831, as he was nearing the end of his apprenticeship, he was sent to help the victims of a cholera outbreak. He became a lifelong student of the disease as a result.
Scientists were convinced at the time that cholera was caused by "miasmas and mephitic vapors." Snow became convinced, however, that the disease was transmitted by something in the water supply. In August 1854, Snow was in London when a large outbreak of cholera occurred in the Golden Square area, a part of the city housing some of London's poorest citizens. By the end of September, 616 persons had died of the disease.
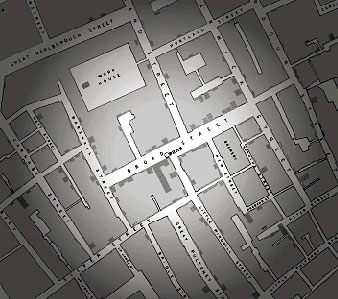
|
|
John Snow mapped a radiating circle of cholera victims and found the now infamous Broad Street pump at the center of the circle. |
Snow was determined to track down the cause of the outbreak. He obtained a list of all the victims and where they had lived from the General Register's office. Using a large map, he marked the residence of every victim who had died during the week ending September 2. The marks on the map revealed that most of the deaths had occurred within a circle with a 250-yard radius, at the center of which was the Broad Street water pump.
At least one of the victims had lived outside the circle, however. So Snow tracked down her survivors and learned that she used to live near the Broad Street pump. She had liked the taste of the water there so well that she often returned to her old neighborhood just for the water. Snow had to conclude that contaminated water was the heart of the problem.
Ultimately, he got permission to remove the handle of the pump so that no one could get drinking water from it, and the outbreak disappeared. Shortly thereafter, workers excavated the pump and found the water to be contaminated with sewage from the adjacent houses.
Snow's methods beautifully illuminate the way science goes about solving problems. His methods furthered the germ theory of disease and established him as something of a hero among modern epidemiologists, who still use his methods of tracking disease today. And if you visit London, you can still see the location of the infamous Broad Street pump. It's marked by red paint on the curb near the John Snow Pub at the corner of Broadwick and Lexington Streets, now in the heart of one of London's more fashionable shopping areas.
The Most Lethal of Strangers
Some infectious diseases appear suddenly or dramatically increase in a population within a short period of time. Scientists refer to this phenomenon as emergence. Medical researchers have identified at least 20 emerging infectious diseases in the past two decades. Some, like toxic shock syndrome and Lyme disease, are a consequence of new products or expanding our habitat. Others are a consequence of evolutionary changes in a specific pathogen, giving it new mechanisms to bypass our immune response (like the Hong Kong influenza virus) or resistance to our antibiotics. Still others, like the hantavirus pulmonary syndrome, are a consequence of a series of environmental factors that offer new opportunities for microbial expansion. Most are among our most dangerous of enemies.
Brief Encounters of the Worst Kind
The microbes responsible for some emerging diseases normally live with other creatures and infect us by accident. Such microbes may not be particularly harmful to their normal hosts, but they are strangers to us—and often our most lethal enemies.
Legionella pneumophila, the bacterium that causes Legionnaires' disease, normally lives with amoebae in fresh water. It infects us when we breathe aerosol droplets of water contaminated with the organism, causing outbreaks like the one at the 1976 American Legion Convention in Philadelphia, where 34 people died and 221 were hospitalized. Scientists found the microbe and its companion amoebae living in the water in one of the hotel's air-conditioning cooling towers.
Even more dramatic are the outbreaks of viral diseases like the mysterious Ebola in northern Zaire and southern Sudan and the hantavirus in the southwestern United States. From their yet-undiscovered hosts, these viruses have spread among people in small, isolated populations, rapidly killing their victims within a few hours to a few days.
Although threatening to us, these outbreaks are often not really successful from the microbes' perspective. The bacteria that cause Legionnaires' disease cannot be transmitted directly from person to person, and so by infecting people, the microbes reach a reproductive dead end.
The Ebola virus can be passed through close contact from person to person, but it often kills its victims so rapidly that it reaches an evolutionary dead end as well— there are no people left to infect. In stark contrast to our "familiar enemies," like S. typhi, this microbe seemingly comes from nowhere, swiftly invades, wreaks devastation, and then disappears.
Ebola virus
 Identity: Virus
Identity: Virus
Residence: Somewhere in Africa,
but no one really knows
Favorite pastime: Still a mystery
Activities: A member of The Lethal
Agents, Ebola has a life cycle that
doesn't usually include humans,
but when it jumps from its still
unknown hosts, it can cause deadly
outbreaks of disease in people;
the virus starred opposite Dustin
Hoffman in Outbreak.
Legionella pneumophila
Identity: Bacterium
Residence: Lives with free-living
amoebae in streams and lakes all
over the world
Favorite pastime: Hanging out in
hot water tanks and air-conditioning
cooling towers
Activities: A member of The
Opportunists, Legionella causes
pneumonia if inhaled in large
enough numbers.
Microbes That Changed History
Emerging infections are not a new phenomenon. Some have changed the course of history.
Plague, or the Black Death, appeared and became epidemic in the cities in western Europe during the Middle Ages. Yersinia pestis, the bacterium responsible for the deadly disease, is transmitted among rats and other rodents by infected fleas. Fleas infesting the brown rats that arrived on the trade ships from the Mideast probably carried the plague bacterium along with them. The native English rat was resistant to the fleas, but the brown rat was more aggressive and quickly outcompeted the locals, thus establishing a new reservoir for the plague bacterium.
People were infected when the hungry fleas feasted on them rather than rats. This provided a great opportunity for the bacteria to expand into a new host, which they did with unbridled enthusiasm. Once infected, some of the people spread the offenders directly from person to person through the air, thereby eliminating the need for the flea intermediary. This was so efficient that over a period of four years, about a quarter of the population in western Europe died from the disease.
The Black Death killed so many people that western European society took a full century to recover. Laborers were in such short supply that wage labor became increasingly attractive. This allowed many people to escape from the feudal restrictions of the time and likely contributed to the demise of the feudal system.
The ability to spread from person to person is a significant advantage for any microbe if it is to survive in a human host. In the case of plague in the Middle Ages, the microbe began infecting people when they came into contact with rats carrying the microbe's host—fleas. The organism's success in finding a new host was magnified when it began to spread directly from person to person. Since people were living in close quarters and in crowded cities by then, the plague bacterium found a grand new opportunity for biologic success.
Other infectious agents have left their mark on civilizations as well. The smallpox virus, introduced into the Americas by Spanish explorers, accounted for the fall of two major empires. Smallpox killed millions of members of both the Aztec and the Incan empires, including their emperors. This made possible the later conquest of those empires by the small armies of Cortez and Pizarro. The English sweating sickness, a mysterious disease that haunted Tudor England from 1485 to 1551, had a similar impact on the population. The disease took the life of Prince Arthur, thus paving the way for his impetuous younger brother Henry to become King Henry VIII. This arguably led to the English Reformation.

|
|
"And I . . . with my own hands, buried five
of my children in a single trench, and
many others did the like. . . . And no
bells rang and nobody wept no matter
what his loss, because almost everyone
expected death. . . . And people said and
believed, 'This is the end of the world.'" |

|
|
A relatively benign relationship among rats, fleas, and Yersinia pestis becomes the Black Death when humans inadvertently find themselves caught in the cycle. |
The hantavirus, in contrast, is not spread from person to person. Outbreaks like that seen in the United States have now been recorded elsewhere, but none have been of the same magnitude as the plague during the Middle Ages. An outbreak in Argentina with a closely related hantavirus has been particularly worrisome, however. Delia Enria, an Argentinian physician and virologist, found evidence of the virus being transmitted from person to person, just as the plague bacterium was. For now, the Argentinian strain of hantavirus that eliminated the mouse intermediary has disappeared, and health-care professionals have breathed a sigh of relief. Events like this still serve to remind us of the small differences that distinguish microbes that infect us.

|
|
A mysterious disease, the English sweating sickness, swept England in the 1500s, then disappeared. Some suspect it might have been caused by a hantavirus. |
As with most new diseases, the emergence of plague or hantavirus is a complex event. The discovery of the factors involved in the emergence of a new disease often reads like a dramatic detective story.
A Pathogen Changes Tactics
It was September of 1996 in Bariloche, a quiet resort town in southern Argentina. And site of another hantavirus outbreak. But this outbreak had a new twist. One of the physicians treating patients in Bariloche became ill. He traveled 1,000 miles to Buenos Aires to see a specialist. The specialist became ill, and then the specialist's wife became ill. All three of them died, and all three were diagnosed with hantavirus pulmonary syndrome.

|
|
Delia Enria |
But the specialist and his wife in Buenos Aires had no known exposure to the rodents that carried the virus, and they had not traveled to the Bariloche region.
Delia Enria, an Argentinian physician and expert in rodent-borne viral diseases, began to suspect human-to-human transmission. This was a phenomenon that had not been seen before with any of the hantaviruses, and the implications, if true, were frightening. It suggested that a new hantavirus had appeared, one that no longer had to rely on its rodent host for transmission.
Enria contacted an old friend and collaborator at the United States' Centers for Disease Control (CDC), C.J. Peters. Although he was skeptical of the story, he realized that if it was true, it was serious. A small CDC team was dispatched to Argentina to help in the investigation. What they found offers us a chilling view of the incredible ingenuity of the microbial world. After ruling out all other means of transmission, Enria and Peters were left with the only possible conclusion. The hantavirus, which they dubbed Andes, had indeed been transmitted from human to human, providing it with a far more efficient means of using us as a major reproductive boost. This meant that avoiding rodents had no meaning, and that the virus could hop on a plane with us and be carried anywhere in the world in a matter of hours.
This time we were lucky. No more cases were discovered. The virus had burnt itself out quickly and then disappeared. But the event serves as a reminder to us that we are locked in a perpetual dance with the microbial world—one in which we do not always have the lead.
A Modern Plague
Twenty-five years ago, another new disease appeared. This disease killed all those who got it, but because the first victims were from the ranks of those stigmatized by society, the disease spread and reached epidemic proportions before we reacted. Acquired immunodeficiency syndrome (AIDS) was a disease that challenged our prejudices, but ultimately this crisis revealed the power of both the political and medical institutions in the United States and elsewhere.

|
|
Germaine Hanquet |
The industrialized nations of the world threw the full weight of their scientific and public health skills into understanding and treating the disease and into controlling the spread of the virus that causes it—the human immunodeficiency virus, or HIV. Although there are still almost a million people infected with the virus in the United States, and another one to two million in Europe, many people think we have contained the epidemic.
But have we? The devastating impact of a new pathogen like HIV emphasizes important differences between the developed and the developing world. At this writing, AIDS has achieved pandemic proportions in sub-Saharan Africa. That level of devastation has not been witnessed since smallpox was introduced into the Aztec population in the 16th century.
In Botswana, the virus has dropped the average life expectancy from 61 years to 47. In Zimbabwe and other countries, one out of every four to five adults is infected with HIV. Of the 30 million people worldwide currently infected with the virus, 26 million reside in one of the 34 sub-Saharan countries. The deadly disease has changed the face of the continent, much as the Black Death changed Europe in the 14th century.
Africa is not alone. Germaine Hanquet, a Belgian physician with Doctors Without Borders, has experienced the differences that economic development can make on two continents. Hanquet saw the effects of HIV while working in Africa. Now she works in Tegucigalpa, the capital of Honduras, where she is translating her experience in Africa into a project targeted at preventing AIDS among the 5,400 street children who live there.
"The reason I'm now here in Honduras working on an AIDS program is that Honduras is a country with a huge AIDS problem. You have 60 to 70% of the cases of AIDS in Central America occurring in a small country which is only 17% of the region. It's urgent to do something. AIDS is affecting the entire country. It's a disease you can prevent, so when you're a doctor, it's a disease you feel you can do something about."
Jumping Species
Scientists now believe that HIV evolved from a similar virus that infects chimpanzees. Named the simian immunodeficiency virus, it does not cause a fatal disease in chimps. We don't know yet whether the virus changed as it changed hosts or whether important differences between the chimps and humans account for its lethal effect on us. Answering that question may give us an important insight into how such an event can occur.
Resourceful Guests
Microbes evolve much faster than we do. Many reproduce very quickly, giving rise to a new generation as often as every 20 minutes. In addition, they mix and match their genetic information on a regular basis. Imagine having a baby every 20 minutes while concurrently passing genes for curly hair to all your family and friends. Within hours, people with curly hair would populate the whole neighborhood. In the microbial world, this "great gene shuffle" regularly gives rise to organisms with new options.
Some reshuffled genes have information that will give microbes an advantage in invading us. These include devices that help them get the food they need, thwart our body's defenses, and fend off our protective microbial inhabitants. For example, E. coli regularly passes the genes for the toxins causing the diarrheal illness we associate with eating undercooked hamburger among its family and friends. And lest vegetarians take too much comfort, scientists have found these gene-carrying toxin producers on everything from apples to lettuce.
A proven approach to sneak past our guard is to use a new disguise. The ubiquitous influenza viruses are a prime example. Mutations and gene shuffling occur regularly, changing the viruses' surface enough so that our immune system doesn't recognize them any more. Such changes vastly delay the action of our immune cells, giving the virus plenty of time to reproduce while making us quite sick.
Another proven approach uses a different form of evasion. We have used antibiotics as an effective way of stopping invading microbes since the discovery of penicillin in the first part of the 20th century. Microbes, in turn, have invoked their evolutionary skills to evade or blunt the killing effects of our antibiotics. By developing resistance to antibiotics, microbes now regularly escape the controlling effects of our best drugs. From the bacteria that cause tuberculosis and wound infection to the virus that causes AIDS, microbes are rapidly eroding the temporary advantage our antibiotics have given us.
Our Evolutionary Dance with Microbes
Even in the face of all the challenges, our success in designing sound prevention and treatment strategies might tempt us into thinking that we will eventually eradicate infection. Such a presumption would be a mistake.
The impact of infectious diseases is still staggering in regions of the world where clean drinking water, uncontaminated food, good nutrition, and health care are not accessible. Globally, infection still ranks among the top five causes of death. And antibiotic resistance is now a common problem. A few microbes, like certain strains of Staphylococcus aureus and Enterococcus, have become virtually untreatable, threatening to return us to an era like the one that existed before the discovery of antibiotics.
This all serves to reminds us that we are in an endless dance with microbes. We are intertwined and we will move and change in concert with each other as long as we humans survive. Microbes are our most intimate and oldest partners. In the end, our efforts to dominate will almost certainly fail. Perhaps new approaches that rely on understanding and entering into their microbial conversations can replace our present strategy to eliminate them. Anticipating and turning aside the most sinister elements of our ongoing tango just might allow us to move forward as true partners, each benefiting from, without harming, the other in life's dance.

|
|
1. Our immune system recognizes, tags, and neutralizes a familiar "flu" virus. |

|
|
2. Through mutation, the outer coat of the virus changes . . . |
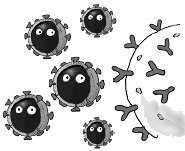
|
|
3. . . . to one that our immune system doesn't recognize. |
This article is excerpted from the book Intimate Strangers: Unseen Life on Earth with the kind permission of its publisher, ASM Press, a division of the American Society for Microbiology. These two pages were adapted from the book's introduction and the rest of the article from a chapter titled "Dangerous Friends and Friendly Enemies." One of the book's coauthors, Mahlon Hoagland, M.D., chaired DMS's Department of Biochemistry from 1967 to 1970 and is currently a member of the Dartmouth Medicine Editorial Board and a trustee of the Hitchcock Foundation. He writes widely about science and in 1982 won the American Medical Writers Association Book Award for Discovery: In Search of DNA's Secrets; his work has appeared in the magazine many times. Cynthia Needham, Ph.D., is an associate professor of microbiology at Boston University and Tufts; Kenneth McPherson is executive producer for science and technology at ICAN Productions, Ltd.; and Bert Dodson, whose work also appears frequently in Dartmouth Medicine, is a noted illustrator with over 70 books to his credit. Intimate Strangers is available at bookstores or www.asmpress.org.
