Deepest Secrets
By Laura Stephenson Carter
Photographs by Medora Hebert (email)
Leslie Henderson and Robert Maue are like a lot of married couples who run a mom-and-pop business together. In their workplace, nearly a dozen workers are busy measuring, mixing, pouring, and boiling colorful ingredients, peering intently at computer screens, or examining tiny objects for imperfections. The soothing strains of a Bach Brandenburg Concerto float from one room, while the lively sounds of Santana's "Supernatural" CD rock another. The mood is cheerful, and an air of excitement pervades the labyrinth of rooms, as some workers joke and laugh with each other while they perform their tasks. Others, however, toil quietly, seemingly oblivious to their surroundings.
But what's different about the "business" that Henderson and Maue run is that their workplace is in the Remsen research building on the Dartmouth Medical School campus in Hanover, they are both associate professors of physiology and of biochemistry, and both have "Ph.D." after their names. They are neuroscientists, and their employees include technicians, Ph.D.'s and M.D.-Ph.D.'s in training, and postgraduate fellows, as well as some undergraduates. Whether they're pipetting homogenized brain cells into tiny vials, creating cell-culture solutions, peering through microscopes, doing computer simulations, or performing molecular-biology techniques like Western blots and polymerase chain reactions, all of them are eagerly engaged in the hunt for the deepest secrets held within the billions of cells that make up a human brain.
Like proud parents (a role that they fill at home, too, with two young sons), Henderson and Maue point out how well the researchers' personalities match their technical skills. Those who enjoy solitude tend to gravitate to the jobs that require them to spend hours working alone—doing things like manipulating cells under a microscope. Others prefer the more social atmosphere that goes along with doing bench research—they can chat with colleagues as they pipette, mix solutions, or perform various complicated procedures.

Bob Maue and Leslie Henderson have been at Dartmouth
Medical School since 1989, studying the basic science
of neural function.
Divining the deepest secrets of the human brain is
painstaking work. Two neuroscientists at Dartmouth
Medical School have devoted their careers to probing
neurons and synapses in an effort to better
understand this most mysterious organ.
It's hard to tell where Maue's lab stops and Henderson's starts—they share space and, occasionally, staff. Veteran lab technician Donna Porter, described by Maue as a "jack-of-all-trades," oversees and assists the researchers in both labs. Maue's researchers —Mark Fry, a postdoc in physiology, and Colleen Paul and Mike Jarvinen, grad students in the molecular and cell biology program—are investigating how specific ion channels in nerve-cell membranes work and how nerve growth factor stimulates neuron growth. Henderson's researchers —graduate students Cassandra Bevan in physiology and Kerry McIntyre in molecular and cell biology, plus M.D.-Ph.D. students Brian Jones and Paul Yang—are exploring how some steroids, and drugs or chemicals that act like steroids, interfere with brain development and affect certain receptors in nerve cells.
Neither Maue nor Henderson had an early interest in neuroscience, but both were initially drawn to animal behavior research. When Henderson was a freshman at Stanford University, she hoped to study chimpanzees with Jane Goodall at the Gombe Reserve in Tanzania.
"Right before I would have gone, there were guerillas—the G-U-E type of guerillas—that kidnapped Jane Goodall and a couple of her students and held them for ransom," Henderson says. "That shut down the program to all the Stanford students."
So Henderson found a way to study animals right on campus—she worked for a researcher who was investigating amphetamine-induced psychosis in rhesus monkeys. She discovered that observing animal behavior under these circumstances didn't interest her as much as she'd expected, but she "became absolutely fascinated with understanding how drugs work in the brain."
She earned a Ph.D. in neuroscience—studying leeches to see how nerve cells make connections— from Stanford in 1982. British neuroscientist Susanna Blackshaw was a postdoc in the same lab and has remained friends with Henderson over the years; she still collaborates today with both Henderson and Maue. "Among Leslie's great qualities," recalls Blackshaw, now a member of the faculty at Oxford, "are her combination of toughness and in- telligence; her direct and straightforward friendliness - there is a 10-year difference in our ages, which I have rarely felt; [and] her experimental skill. It is great fun to work with her at the bench."
Henderson went on to do postdoctoral training at the University of California-San Diego. It was there that she met Maue, who was working on a Ph.D. in physiology-pharmacology. With broad interests and an insatiable curiosity about the way things work, Maue had been attracted to U.C.-San Diego because of its intriguing research. "There were people who did respiratory studies on Mt. Everest," he says, "riding Exercycles at 20,000 feet." And he was really interested in research at U.C.- San Diego's Scripps Institute of Oceanography on the diving behavior of seals and penguins. "I got to go to the South Pole for six months," he says, "chasing seals around and trying to understand how they survived down there." He wanted to know how seals could hold their breath for so long, how they could live in a place that's so cold and dark six months of the year, and what they ate.

|

|
|
|
|
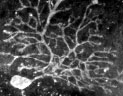
At right, grad student Kerry McIntyre and technician Donna Porter work together at the bench. Above are further magnifications of Purkinje cells (see the box on the facing page for details) - one of Bob Maue's research interests. The top image shows rows of round Purkinje cells in the folds of cerebellar tissue, plus a more diffuse layer of dendrites. The bottom, higher-magnification image shows an individual Purkinje cell and its extensive dendritic branches. |
|
When he returned from Antarctica, Maue began to work with a professor who became his advisor and friend - Vincent Dionne. Dionne was investigating the cellular basis of the sense of smell and wanted to measure electrical signals that olfactory neurons generate in response to odors. "Nobody had ever done that," Maue says. Nevertheless, Maue mastered the difficult and demanding technique.
"Bob is willing to tackle problems that seem daunting to others," says Dionne, who's now a professor of biology and director of graduate studies at Boston University's Marine Program in Woods Hole, Mass. "He was one of the first two people who took patch-clamp recordings from olfactory neurons" when this technology for recording electrical signals from nerve cells was in its infancy. Maue, he says, had and still has "remarkable persistence."
In 1985, Henderson and Maue married and, the following year, moved to Boston to do postdoctoral work. It was a second postdoc for Henderson, and she worked at Tufts with neuroscientist Paul Brehm. Maue, after a one-year fellowship at Brandeis, worked at Tufts's New England Medical Center with neuroscientist-molecular biologist Gail Mandel, who, coincidentally, was Brehm's wife. Mandel and Brehm, who are now at SUNY-Stony Brook, are one of several neuroscience faculty couples whom Henderson and Maue have gotten to know over the years.
At that point, Maue was a classically trained physiologist, but he soon became a skilled molecular biologist after working in Mandel's lab. They were among the first to clone genes that encode proteins called sodium ion channels. "These ion channels - you can imagine these molecules are shaped like a donut or a grommet - undergo slight rearrangements when they open and form a small 'hole' in their center," says Maue. "When the channel is open it forms a pathway [so] ions can flow across the membrane."
There are hundreds of types of ion channels in cell membranes - ions can race through a single channel at the rate of 100 million ions per second - but the most important ones for nerve cells are those that regulate the flow of potassium, chloride, calcium, and sodium ions. Electrical signals in nerve cells, known as "action potentials," are generated by a sudden influx of sodium ions through voltage-sensitive sodium ion channels. This work has broad clinical relevance, for mutations in the genes encoding sodium channels can have devastating consequences, including skeletal muscle disorders, cardiac arrhythmias, and epileptic seizures.
Maue also worked on growth factors, including nerve growth factor (NGF), in Mandel's lab. "At the time," says Maue, "very little was known about how these growth factors influenced neurons - how they helped them grow, how they helped them make contacts with their targets, how they helped them stay alive, and especially," he adds, "what role they played in governing ion channel expression."
As is common, Maue brought some of his post-doctoral work to Dartmouth when he came to Hanover in 1989. He has since expanded his investigations of how growth factors affect voltage-dependent sodium-channel genes. "There's lots of aspects of how neurons develop," Maue says. "It's like building a house. I could tell you I'm interested in how houses are built, and you'd say, 'Well, what part - plumbing, electrical, heating?'" He's interested in understanding how the voltage-dependent sodium channels in neurons are assembled, what controls their activity, how growth factors are involved, and how modifying some of the genes that encode sodium channels might make a difference in a neuron's pattern of activity.
"The 'what difference does it make' part is the hard part," Maue says. "One approach we're using is viral-mediated gene delivery . . . to infect nerve cells and express ion-channel genes. We can turn one on when it's not supposed to be [on] and see what happens. We put one in a different place in the cell and see how that changes things."
Mike Jarvinen and Mark Fry are being trained as classical physiologists and molecular biologists, just as Maue was. It's still fairly unusual to find researchers who are skilled in both areas. They can measure electrical signals from nerve cells using the patch-clamp recording technique that Maue used in his doctoral research, then do molecular work on the same cells (see "Listening to cells talk" at the bottom of the page for a glimpse of what this sort of work involves).
In 1989, Henderson also brought to Dartmouth her postdoctoral work - on neuromuscular synapses in frogs. Research techniques have evolved since then, and now researchers can study synapses between brain neurons, too. Still fascinated by how drugs work in the brain, she's trying to understand how anabolic steroids affect an ion channel called a GABA(A) receptor.
GABA (gamma-aminobutyric acid) is an amino acid with neurotransmitter activity, and the GABA(A) receptor is a type of ion channel that regulates the flow of chloride ions into and out of the nerve cell. Chloride ions inhibit nerve-cell activity because they prevent sodium channels from generating the electrical signals that travel along neurons. A myriad of psychoactive drugs - sedatives, antianxiety drugs, barbituates, anesthetics, as well as synthetic and naturally-occurring neurosteroids - can bind to the GABA(A) receptor and change the way it functions. "Many of these drugs increase chloride flow through the GABA(A) receptor, and that's why these are sedatives and hypnotics and antianxiety drugs," Henderson says. "They basically dampen activity in the central nervous system."
Anabolic androgenic steroids - also referred to as "performance-enhancing drugs," because people take them to improve athletic performance - alter the GABA(A) receptors, too. They interfere with nerve-cell activity, seem to affect brain development, and may even cause permanent and irreversible damage to the areas of the brain that regulate the reproductive system - especially in women. Henderson is concerned about reports showing that the biggest increase in anabolic steroid abuse is among junior-high and high-school students - girls in particular.
|

|
|
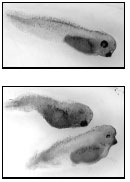
Brian Jones, at right, is an M.D. - Ph.D. student in Leslie Henderson's lab. Among the studies ongoing there is one looking at the effect of endocrine-disrupting compounds (EDCs) on the development of the neural crest of tadpoles. Above, the top image shows a normal tadpole and the bottom image a pair of tadpoles after 48 hours' exposure to an EDC; note the latters' crooked tails and poorly differentiated eyes. |
|
"There is a lot of emphasis in our society on - even if you aren't necessarily athletic - looking athletically fit," Henderson says. "A lot of kids - even those who aren't in sports - are taking these drugs. What really worries people like me [is that] we have almost no idea of what they're doing in the brain. We know they damage the liver. We know they damage the heart. But what they do in the brain, in terms of mechanisms, is a mystery. Kids [are] taking them at a time when they're going through puberty, when their sensitivity to hormones is heightened to begin with.
"We're interested in parts of the brain that regulate reproductive behaviors, sexual behaviors, and maternal behaviors," explains Henderson, whose particular interest is neurons in the hypothalamus. Recent studies suggest that steroids may affect the fertility and reproductive senescence (the time of menopause's onset) in people who took steroids as children - some as young as 13 and 14 - even though they later stopped. The steroids are also believed to cause changes in anxiety and in aggression. "There have been cases where people have gone out and killed someone," says Henderson, "and they've used the legal excuse that they have steroid rage from taking these anabolic steroids." In addition, some steroid abusers become so obsessed with their body shapes, she adds, that even "hugely puffed up people view themselves as little and scrawny, so they keep abusing."
Brian Jones, Paul Yang, and Kerry McIntyre are responsible for gathering information for the GABA(A) receptor project; they record signals from neurons, do computer simulations, and perform sophisticated molecular-biology techniques, such as reverse-transcriptase polymerase chain reactions. So far, Henderson says, "we know that the steroids affect GABA(A) subunit receptor gene expression [and that] they don't do the same thing in different brain regions."
Henderson and graduate student Cassandra Bevan are also looking at how endocrine-disrupting compounds - like herbicides, pesticides, and some by-products from manufacturing - interfere with the development of the nervous system. "Obviously, the most famous one is DDT, which was studied by Rachel Carson in Silent Spring," Henderson says. "We know, for example, that DDT completely messes up reproduction. It's a real issue right now for the government, because they have something like 86,000 chemicals that may be potential endocrine disruptors. That's an awful lot of drugs to try and screen."
Henderson tests suspect compounds on Xenopus frog tadpoles. The Xenopus frog, probably the most common animal model for embryology and developmental studies, can produce several thousand eggs daily, which become free-swimming tadpoles in a couple of days. "You don't have to wait very long to see the effects," says Henderson.
No one yet understands the long-term effects of exposure to these chemicals, but Henderson suspects that they interfere with areas of the brain that control reproductive behaviors. "There's a major shift in the onset of puberty in young girls," she says. "Is that coming from exposure to chemicals during early development?" Also, she adds, "there are definite decreases in sperm count in men in First-World, industrialized countries. Is that a facet of being exposed to these chemicals? We don't know for sure yet. What is clear is that developing organisms are much more sensitive to steroid effects than are adults. So by looking at these tadpoles, we may get a handle on things that we can't study in humans."
Tadpoles that have been exposed to endocrine disruptors do not develop properly. Their muscle cells are malformed, there's no dorsal fin, and the pigment cells — which arise from a structure called the neural crest — don't develop. "Pigment cells are easy sentinels that we can use to see that there is something wrong with the neural crest lineage," Henderson explains.
Neural crest cells, a cap of tissue in the head of the tadpole embryo, give rise to the ganglia that innervate the head as well as the peripheral nervous system. "It turns out that one of the things that you need to get neural-crest development are growth factors, including NGF," she says. Neurons taken from early-stage tadpoles and grown in culture will respond positively to NGF. Neurons that have been exposed to endocrine disruptors will survive and don't look any different from normal neurons. But endocrine disruptors will block the ability of NGF to induce differentiation in these neurons.
In addition to focusing on their own work, Maue and Henderson often collaborate with other labs at Dartmouth. They have recently been working with T.Y. Chang, Ph.D., chair of biochemistry, on a study of Niemann-Pick disease, a neurodegenerative disorder characterized by an inherited inability to process cholesterol.
"This Niemann-Pick project was a perfect example of the progress that can be made by collaboration," says Maue. "T.Y. Chang has done internationally recognized research on cholesterol. We've spent our careers studying nerve cells. While people had looked at the effects of the disease in brain slices post mortem, nobody had looked at live neurons affected by Niemann-Pick disease."
|
|
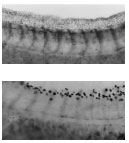
Above are microscopic views of the tails of the tadpoles pictured on the facing page. The tail of the normal tadpole (top) is relatively straight and shows a well-defined fin on the upper surface, numerous small pigment cells in the fin, and well-differentiated pigment cells just above the tail muscles (the chevron-like structures). But in the tadpole exposed to an EDC (bottom), the back is abnormally curved, the dorsal fin and its pigment cells are completely missing, and the pigment cells atop the tail muscles are poorly differentiated. This work has implications for how EDCs—such as herbicides and pesticides—affect humans. |
Maue's group discovered that brain cells from Niemann-Pick mice—a strain with this genetic disorder—could neither handle cholesterol properly nor respond normally to nerve growth factor. The concept that Niemann- Pick nerve cells couldn't respond to growth factors was "a new idea in the Niemann-Pick field of research," says Maue. This clinically relevant aspect of his work has received considerable attention; it holds potential as a therapeutic agent for treating neurodegenerative diseases such as Alzheimer's, Parkinson's, Huntington's, and amyotrophic lateral sclerosis, known as Lou Gehrig's disease.
"And it's a very topical idea," explains Henderson. "It has ramifications beyond Niemann-Pick disease," in that it raises some fundamental questions about the interactions of cholesterol metabolism and nerve-cell function.
The resulting article, published in the Journal of Biochemistry, won the distinction of being selected as an "Editor's Choice" in the July 2000 issue of the prestigious journal Science.
Henderson also works closely with Ann Clark, Ph.D., an associate professor of psychological and brain sciences at Dartmouth, who is investigating the effects of anabolic steroids on puberty, on the display of female sexual behaviors, and on cognitive and emotional behaviors in adult rats. "I study whole animals—observable behavior—while Leslie does more microscopic/molecular analysis," says Clark.
What led Henderson and Maue to study the brain? "In some ways it's egotistical," says Henderson. "We all want to know why we are human, and certainly it's your brain that makes you different from other things as opposed to your kidney or your heart." And, she adds, "I think there is a huge interest, as the baby boom generation ages, in understanding neuroprocessing—because we're all scared to death that we're going to live to be 110 but we'll have Alzheimer's and we won't have any idea what's going on. Or we need to take care of parents . . . in that situation. People have always, obviously, been worried about their children, but the heightened awareness of what we're doing in terms of the environment and the impact it may have on development is now commonplace. It's not just people in science who recognize that—it's anybody who picks up Time magazine."
Maue had his own reasons for gravitating to neuroscience. "Everybody has their own 'black box,'" he says. While some people are interested in studying whole animals, others "think about the heart or the kidney or the liver, and so they're thinking about an organ or tissue. For me, a nerve cell is my 'black box.' When I close my eyes, that's what I think about—the molecules in it, where they're put, what kinds of signals it makes, how the cell grows. I tell my students, 'There's always a smaller level.' You can take it down to atoms and particles. . . . I think the level you end up working on in science is a personal decision. What kinds of questions, what kinds of answers, do you find the most satisfying. Some people like to look at cells. Some like molecules. Some people don't get excited by electrical signals on a computer screen, while others aren't turned on by bands on a gel. It depends on what you find personally satisfying, what size 'box' you like thinking about."
But, he adds, "even with all the recent progress that's been made, we know so little about how the brain works. . . . You hear a tremendous amount about Alzheimer's disease and Parkinson's disease and all sorts of neurodegenerative diseases. These are where populations of nerve cells in the brain are slowly dying. In most cases, we don't know why that happens. There's a tremendous effort, both in research and in the private sector, to figure out how to halt that, to prevent that."
Henderson echoes Maue's assessment of the field to which both have devoted their careers, agreeing that there has been considerable progress but that much remains to be done. "Fifty years ago, you could make inferences, but you couldn't study things," she says. "Now, at a molecular level, a single-cell physiology level, and with imaging studies, you can really begin to put things together . . . to understand how the brain works."
But, she adds, stretching her arms wide to suggest how long it will be until all the brain's secrets have been divined, "I think on a timeline that it's this far. And," she concludes, gesturing to a spot near the very beginning of the span she's indicated, "we're still over here."
"Listening to cells talk"
Watching Mike Jarvinen at work is an enthralling experience. First, he places thin slices of mouse cerebellum, bathed in an oxygenated saline solution, into a petri dish. Then he carries the sample over to a microscope and sits down in a cockpit-like area surrounded by high-tech electronic equipment - to his left, a computer monitor, a video monitor, and an oscilloscope are stacked so he can keep an eye on all three screens while he's working on the neurons under the microscope. After setting the petri dish in place, he manipulates the microscope's knobs to focus in on a large neuron called a Purkinje cell. Purkinje cells are one of five types of neurons in the cerebellum and play an important role in motor skills and coordination.
A camera mounted above the microscope captures the live image and projects it onto a video screen. Jarvinen gently spins a dial to lower what he calls an electrode - it's really a glass micropipette with a micro-thin metal filament inside - until it just barely touches a Purkinje cell. To keep cellular debris from clogging the top of the micropipette, Jarvinen blows air gently into a long tube that's connected to the electrode.

|
|
Above, Jarvinen keeps his eye on a stack of monitors while controlling the micropipette. Below is the image on his video screen; the pipette's tip is visible at the top of this slice of mouse cerebellum, and the line it's touching is the Purkinje cell layer. |
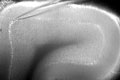
|
Bob Maue, who's also watching Jarvinen work, whispers that the process is like using a broomstick to touch a basketball floating in a swimming pool. "You have only one try," he adds. Either the electrode successfully records the cell's electrical signals, or it irrevocably damages the cell.
Jarvinen then sips air from the tube to tighten the seal between the micropipette and the cell membrane. Being a musician may be helpful - he plays the trumpet in the Dartmouth Symphony Orchestra and in the Lyme Town Band - in knowing how to apply just the right amount of pressure. But even someone with a lot of experience with the technique will typically get a recording from only two or three cells out of 30 tries.
Success! Jarvinen describes recording the electrical signals as "listening to the cells talk." Each signal is represented by a jagged line that appears on the oscilloscope screen.
Next, he carefully suctions the cell into the micropipette and squirts it into a test tube that he sets aside for later genetic analysis; the cell doesn't have to be alive for the additional testing. Jarvinen works tirelessly for several hours until the brain slice gets too old and neurons begin to die.
The pattern of electrical signals emanating from the Purkinje neurons varies according to the mouse's age. In four-day-old mice, Purkinje cells produce five action potentials per second, while in 21-day-old mice, the same type of neurons produce 20 to 40 signals per second.
"So far, there do appear to be changes in the expression of different sodium-channel genes that correlate with the changes in activity," Maue says. Up to now, Jarvinen has been examining neurons from normal mice. Eventually, Maue plans to have his researchers examine neurons from mutant mice that are missing the nerve growth factor that regulates the sodium-channel genes.
Laura Carter is the associate editor of Dartmouth Medicine.
